
Guide ROTEM
®
Analysis - 09-2016 2
Introduction
In this text, the basis of ROTEM
®
analysis is described together with its
use during the management of acute bleeding.
The management of acute bleeding is a complex challenge. Little that is
done in this area is fulfilling the hard criteria of evidence based medicine.
The recommendations in this compendium are based on the experience
of the authors and on the discussion with centres, which use the
ROTEM
®
system in clinical routine. However, these recommendations
have not yet been prospectively validated.
Causes of Haemostasis Disorders
Haemostasis disorders can have several causes. Rather chronic
processes (such as comorbidities of the haemostasis-related organs
liver - kidney - bone marrow) or hereditary diseases can be differentiated
from more acute alterations due to trauma, haemodilution and the
current treatment. The resulting alterations affect the plasmatic
coagulation factors, platelets and the fibrinolytic system.
Bleeding most frequently occurs during and after surgical interventions
or traumas, i.e. in situations where trauma and secondary alterations
(e.g. due to haemodilution) are added to the disposition of the patient.
During such complex haemostasis disorders, the clinical significance of
the routine parameters PT, aPTT and platelet count is rather weak. This
leads to the interest in laboratory methods, which better reflect
haemostasis during these complex processes.
A. Calatzis, W. Schramm, M. Spannagl. Management of Bleeding in Surgery and
Intensive Care. I. Scharrer / W. Schramm (Ed.), 31 st Hemophilia Symposium
Hamburg 2000, Springer Verlag Berlin Heidelberg 2002.

3 Guide ROTEM
®
Analysis - 09-2016
Alterations of haemostatic causes
Trauma and coagulopathy as a cause of bleeding

Guide ROTEM
®
Analysis - 09-2016 4
Thromboelastometry
Dr. Andreas Calatzis, Prof. Dr. Michael Spannagl, Dr. Matthias Vorweg
Targeted Treatment of Bleeding Events
During acute bleeding, a multitude of different therapeutic options are at
disposition to the physician. The difficulty is to choose the right
medication at the right time and to evaluate how much, respectively how
often, the respective therapeutic option has to be applied. Typically, only
the
right therapy will stop the bleeding. It will be of little use to the patient,
if he is transfused with FFP while he is bleeding because of
thrombocytopenia or hyperfibrinolysis. Although this sounds self-
evident, in the clinical everyday routine, a "blind" therapy is often applied.
This means that different medication and blood products are
administered consecutively until the bleeding stops. If the cause of the
bleeding is not the most obvious, unnecessary medication and blood
products are administered. Thus, unnecessary costs are created and the
patient is exposed to potentially harmful preparations.
TEG / ROTEM
®
- History
Thrombelastography was developed during world war II by professor H.
Hartert in Heidelberg. Following a quite broad application in the 50's and
60's, the interest in TEG decreased in the 70's. In the 80's it came to a
renaissance of TEG, especially in the United States, because of the
application in anaesthesia for the management of acute bleeding. The
ROTEM
®
system is an enhancement of thrombelastography and was
developed during 1995-1997 in Munich. The instrument includes four
measurement channels for simultaneous determinations, an integrated
computer for automatic analysis and an electronic pipette for interactive
test operation.
Note: The term "TEG" was introduced by Hartert in his first publication
on thrombelastography in 1948. Surprisingly, in 1993, an American
company obtained a trade mark on this term in the USA, after 45 years
of its use as a generic medical term. In order to achieve a global
uniformity of the name, the manufacturer of the ROTEM
®
system (Tem
Innovations GmbH, Munich) has renamed its instrument from "ROTEG"
into "ROTEM" and the tests accordingly from "EXTEG" into "EXTEM",
"INTEG" into "INTEM" etc. in 2003. "TEM" thereby stands for
"thromboelastometry" (analogous to the term "thromboelastography"),
thus the plotting of the clot firmness.

5 Guide ROTEM
®
Analysis - 09-2016
Bleeding: Therapeutical options
ROTEM
®
Thromboelastometry system
• 4 channels for simultaneous assays
• Automated testing in ROTEM
®
sigma, standardized
electronic pipetting for ROTEM
®
delta
ROTEM
®
delta ROTEM
®
sigma
Guide ROTEM
®
Analysis - 09-2016 6
ROTEM
®
thromboelastometry detection method
In the ROTEM
®
system, the sample is placed into a cuvette and a
cylindrical pin is immersed. Between pin and cuvette remains a gap of 1
mm, which is bridged by the blood or the blood clot. The pin is rotated by
a spring alternating to the right and the left. As long as the blood is liquid,
this movement is unrestricted. As soon as the blood clots, the clot
restricts the rotation of the pin increasingly with rising clot firmness.
Thus, the rotation of the pin is inverse proportional to the clot firmness.
It is detected optically. An integrated computer calculates the ROTEM
®
curve as well as its numerical parameters.
In contrast, in the TEG according to Hartert, the cuvette is rotated. The
pin is suspended freely from a thin wire and does not move until a clot
forms. Because of this free suspension of the pin, the TEG according to
Hartert is quite susceptible to vibration and mechanical shocks.
Due to the mechanical measurement principle of ROTEM
®
analysis,
blood or plasma can be analysed likewise. This is advantageous for the
point-of-care application, as centrifugation of the sample is omitted
there.
The parameters of ROTEM
®
thromboelastometry analysis
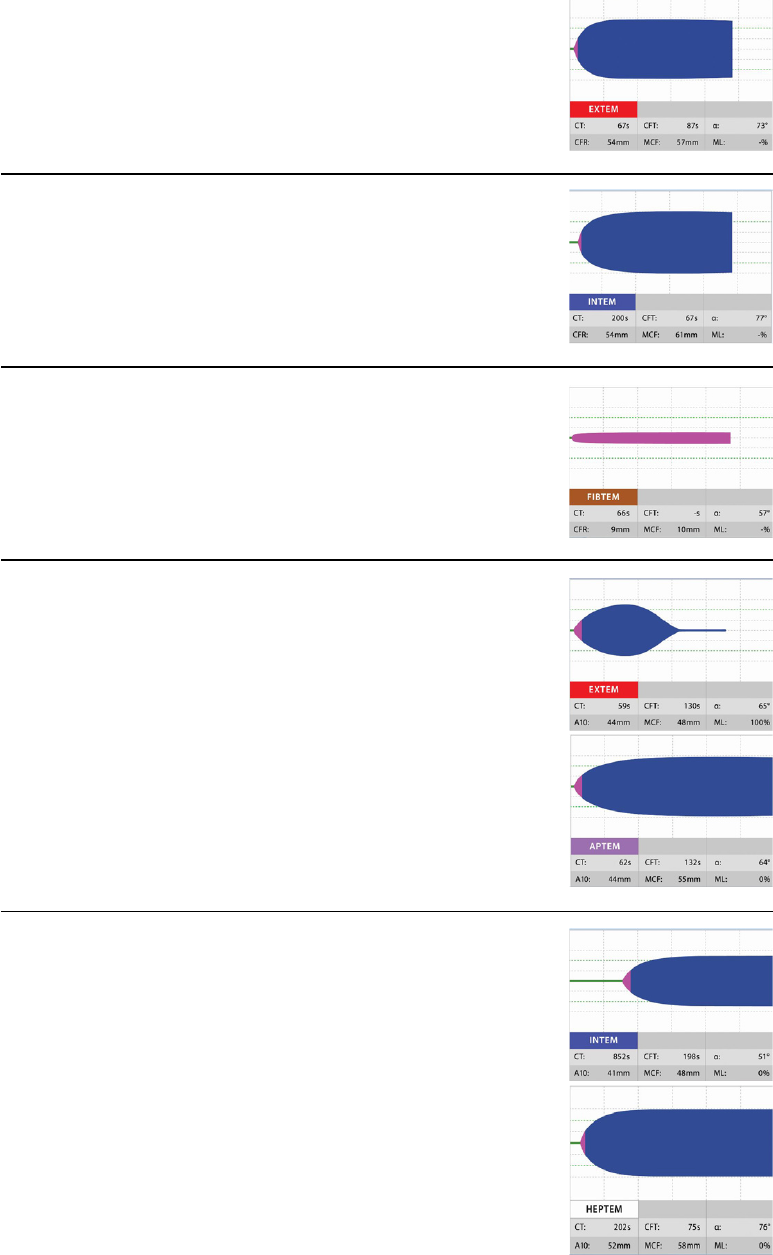
For historical reasons, the curve is plotted two-sided, expressed in mm.
CT (clotting time): time from start of the measurement until
initiation of clotting
initiation of clotting, thrombin
formation, start of clot polymerisation
CFT (clot formation time): time from initiation of clotting until a clot
firmness of 20 mm is detected
fibrin polymerisation,
stabilisation of the clot with thrombocytes and FXIII
MCF
(maximum clot firmness): firmness of the clot
increasing
stabilisation of the clot by the polymerised fibrin,
thrombocytes as well as FXIII
ML (maximum lysis): reduction of clot firmness after MCF in
relation to MCF
stability of the clot (ML< 15%) or
fibrinolysis (ML > 15% within 1h)

7 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
thromboelastometry detection method
ROTEM
®
thromboelastometry parameters and scaling

Guide ROTEM
®
Analysis - 09-2016 8
ROTEM
®
thromboelastometry tests
In the past, “the thrombelastogram“ was analysed using freshly drawn
blood without the addition of any citrate / calcium and without any
activators. The measurements were therefore very time consuming (45 -
60 min.) and quite unspecific.
With the ROTEM
®
, activated determinations are usually performed. As
in the laboratory coagulation analysis, various activators or inhibitors are
added to the sample, in order to represent different processes of
haemostasis. For the analysis, citrated blood is usually used.
In EXTEM, coagulation is activated by a small amount of tissue
thromboplastin (tissue factor). This typically leads to the initiation of clot
formation within 70 seconds. Thus, clot formation can be assessed
within 10 minutes.
In INTEM, coagulation is activated via the contact phase (as in the aPTT
and ACT). The INTEM is therefore sensitive for factor deficiencies of the
intrinsic system (e.g. FVIII) and for the presence of heparin in the
sample.
In FIBTEM, coagulation is activated as in EXTEM. By the addition of
cytochalasin D, the thrombocytes are blocked. The resulting clot is
therefore only depending on fibrin formation and fibrin polymerisation.
In APTEM, coagulation is also activated as in EXTEM. By the addition of
aprotinin or tranexamic acid in the reagent, fibrinolytic processes are
inhibited in vitro. The comparison of EXTEM and APTEM allows for a
rapid detection of fibrinolysis. Furthermore, APTEM enables the
estimation if an antifibrinolytic therapy alone normalises the coagulation
or if additional measures have to be taken (e.g. administration of
fibrinogen).
In HEPTEM, coagulation is activated as in INTEM. The addition of
heparinase in the reagent degrades heparin present in the sample and
therefore allows the ROTEM
®
analysis in heparinised samples.
Reagent type Test name for each reagent type
Single Use EXTEM S FIBTEM S APTEM S INTEM S HEPTEM S
Liquid EXTEM (L) FIBTEM APTEM INTEM HEPTEM
Cartridge EXTEM C FIBTEM C APTEM C INTEM C HEPTEM C
9 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
thromboelastometry tests
EXTEM (L, S, C): activation of clot formation by
thromboplastin (tissue factor).
Assessment of factors VII, X, V, II, I, platelets,
fibrinolysis
INTEM (S, C): activation of clot formation via the
contact phase.
Assessment of factors XII, XI, IX, VIII, X, V, II, I,
platelets, fibrinolysis
FIBTEM (S, C): activation as in EXTEM with the
addition of cytochalasin D, a platelet blocking
substance. In the FIBTEM assay, fibrinogen
levels and fibrin polymerisation can be assessed
in a functional way.
APTEM (S, C): activation as in EXTEM with the
addition of aprotinin or tranexamic acid,
fibrinolysis inhibitors. In an assay comparing
APTEM to EXTEM, fulminant hyperfibrinolysis
can be recognised within 10-20 minutes.
HEPTEM (S, C): activation as in INTEM with the
addition of heparinase. Heparinase degrades
heparin. When HEPTEM results are compared
to INTEM, heparin related coagulation
disturbances can be specifically detected.
Guide ROTEM
®
Analysis - 09-2016 10
ROTEM
®
thromboelastometry expected values
On the opposite page, typical ROTEM
®
thromboelastometry values are
shown, which are found when healthy patients without coagulation
disorders are analysed. Depending on the examined population, these
values can vary (for example, when healthy younger persons are
assessed, lower MCF values are found). It is therefore recommended, at
introduction of ROTEM
®
, to analyse some patients without pathological
findings in order to establish respective 'local' reference ranges.
Interpretation of HEPTEM / APTEM / FIBTEM
In HEPTEM and APTEM, the comparison with INTEM respectively
EXTEM is important for the interpretation. A shortening of the clotting
time in HEPTEM as compared to INTEM indicates a heparin effect.
The ’spindle’ shape of the TEMogram in the EXTEM, INTEM or FIBTEM
assays gives an indication of fibrinolysis. The reversal back to a normal
TEMogram shape in the APTEM assay confirms the fibrinolysis and
allows to judge the patient‘s clot quality after an optional hyperfibrinolytic
treatment.
A reduced MCF in FIBTEM indicates a reduced fibrinogen level and / or
a clot polymerisation inhibition. Discrepancies between FIBTEM and the
fibrinogen determination in the laboratory are frequently found, as
FIBTEM is much more sensitive to clot polymerisation disorders than
conventional laboratory assays.
Classification of the ROTEM
®
results
The lower right table shows an orientating classification of the ROTEM
®
results based on our clinical experiences.
Depending on the situation and possible comorbidities of the patient,
different target ranges will be aimed for the MCF, respectively CFT.
During surgery, we typically aim for a MCF value of at least 40 mm and
a CFT of maximal 300 s. In persistent bleeding situations, an almost
normalisation of the ROTEM
®
findings will be aimed for.
Hyperfibrinolysis (lysis of the clot in vitro) is always pathological and can
be treated with an antifibrinolytic drug. Nevertheless, hyperfibrinolysis
can be self-limiting, which can be checked by repeated determinations
without any preceding therapy.

11 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
thromboelastometry reference ranges
(Lang et al. 2006 for ROTEM
®
delta, preliminary for ROTEM
®
sigma)
*Historical value
INTEM (S) / EXTEM (S) results - Clinical interpretation
MCF
MCF > 72 mm: enhanced haemostatic reserve
MCF 50-72 mm: normal range
MCF 46-49 mm: usually unimpaired haemostasis with reduced reserve
MCF 40-45 mm: bleeding risk
MCF 30-39 mm: high bleeding risk
MCF < 30 mm: usually no effective haemostasis
CFT
CFT 34-159 s: normal range
CFT 160-220 s: usually unimpaired haemostasis with reduced reserve
CFT 221-300 s: bleeding risk
CFT 301-400 s: high bleeding risk
CFT > 400 s: usually no effective haemostasis
Fibrinolysis
Lysis of the clot within 20 minutes (fulminant lysis): usually acute
bleeding. Lysis of the clot within 20 – 40 minutes: high bleeding risk.
Lysis of the clot after more than 40 minutes: frequently clinically
insignificant, may however raise to fulminant lysis.
Test
/ Parameter
CT
(s)
CFT
(s)
A10
(mm)
MCF
(mm)
LI60
(%)
EXTEM (S) 38-79 34-159 43-65 50-72 85*
EXTEM C 50-80 46-149 43-63 55-72 94
INTEM (S) 100-240 30-110 44-66 50-71 85*
INTEM C 161-204 62-130 43-62 51-69 87
HEPTEM (S, C)
Comparison with INTEM (S,C). A better clot formation in HEPTEM (S,C) as
compared to INTEM (S,C) indicates the presence of heparin or heparin-like
anticoagulants in the sample.
APTEM (S, C)
Comparison with APTEM (S,C). A better clot formation in APTEM (S,C) as
compared to EXTEM (S,C) is a sign of hyperfibrinolysis.
FIBTEM (S) n.d. n.d. 7-23 9-25 n.d.
FIBTEM C n.d. n.d. 6-21 6-21 89
Guide ROTEM
®
Analysis - 09-2016 12
Assessment of the ROTEM
®
thromboelastometry analysis
The ROTEM
®
analysis covers the whole process of whole blood
coagulation, from the formation of the first fibrin strands over the
maximum firmness of the clot until its lysis.
The assessment of the ROTEM
®
analysis is carried out along the time
axis (from left to right): A disturbed activation of coagulation is indicated
by a prolonged clotting time. As causes, a factor deficiency or a heparin
effect have to be considered. The comparison of INTEM and HEPTEM
allows for a specific detection of a heparin effect.
An abnormal clot formation is indicated by a prolonged clot formation
time (CFT) and/or a reduced clot firmness (A10/MCF). The CFT is
thereby influenced stronger by a clot polymerisation disorder than the
MCF.
A prolonged CFT with at the same time normal A10/MCF indicates
therefore a polymerisation disorder, whereas a reduced A10/MCF with a
normal CFT rather indicates a deficiency of clottable substrate
(fibrinogen and/or platelets).
Fibrinolysis is detected by the lysis of the clot (ML > 15%) or by the
finding of a better clot formation (shorter CFT, greater MCF) in APTEM
(S,C) as compared to EXTEM (S,C). If in APTEM (S,C), with the
occurrence of the typical pattern of a hyperfibrinolysis (spindle shaped,
total lysis of the clot firmness) in EXTEM (S,C) (as in INTEM (S,C),
FIBTEM (S,C), HEPTEM (S,C)), the hyperfibrinolysis is not present, then
hyperfibrinolysis is confirmed.
Limitations
In the interpretation of ROTEM
®
analysis it is important to know and
consider the limitations of the method. The ROTEM
®
delta and sigma
tests are not sensitive to the effect of the platelet inhibitors Aspirin
®
,
clopidogrel and Reopro
®
(only in supra-therapeutic doses). In this
situation, ROTEM
®
platelet analysis should be performed. Also, the
effect of the von Willebrand factor is not detected. Furthermore, a normal
ROTEM
®
analysis does not exclude the anticoagulants Orgaran
®
,
pentasaccharide, low-molecular-weight heparin as well as oral
anticoagulants such as Warfarin
®
. For analysis of these factors, other
diagnostic tests have to be performed.

13 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
thromboelastometry: detection and therapy
Limitations of ROTEM
®
thromboelastometry
Platelet inhibitors:
• no detection of Aspirin
®
• no detection of clopidogrel/Plavix
®
• no detection of von Willebrand syndrome
• poor sensitivity to Reopro
®
Anticoagulants:
• poor sensitivity to low molecular weight heparin, Orgaran
®
and
pentasaccharide
• poor sensitivity to oral anticoagulants
(coumarins: Warfarin
®
, etc.)
Activation of coagulation
=> protamin, FFP of PCC
=> differentiation with HEPTEM
Clot formation
=> infusion of platelets and/or fibrinogen/
FFP
=> differentiation with FIBTEM
Fibrinolysis
=> infusion of antifibrinolytics
=> more rapid detection with the
combination of APTEM-EXTEM
Consequence:
• Combine with other methods (e.g. ROTEM
®
platelet
aggregation) where required.
• Consider limitations for interpretation!
Guide ROTEM
®
Analysis - 09-2016 14
Performance of ROTEM
®
thromboelastometry analysis
As in all diagnostic tests, correct pre-analytics and correct performance
of the assay are essential for meaningful results.
As ROTEM
®
is run directly with citrated whole blood, a specific sample
preparation is not necessary. “Correct sampling“ means: Complete filling
of the sampling tube (in order to ensure the correct citrate-blood ratio);
the assurance, during sampling from catheters, that no contamination
with heparin or other anticoagulants occurs; and the avoidance of
haemolysis during sampling (prevent excessive stasis, use of a needle
with sufficiently wide diameter). We typically aim for an analysis of the
sample within 2 hours from the sampling of the blood
(if required up to four hours). The analysis of samples that have been
transported by a tube system is usually possible. As a precaution this
should be verified (split the sample and analyse with/without transport by
the tube system).
The steps to be performed for ROTEM
®
delta analysis are shown on the
right. The test operation is generally simple - also for staff without any
laboratory experience. Nevertheless, a certain familiarisation period and
motivation are necessary.
The ROTEM
®
sigma offers a fully automated, cartridge based system.
The familiarisation is reduced to a minimum.
Apart from the correct performance of the analysis – as in every
laboratory test – the plausibility control of the analysis is important.
Measurements with irregular shapes (steep rise or fall of the clot
firmness, noised curves, and start of the clot formation in less than 20
seconds), generally accompanied by error messages, should be
repeated.
When using liquid reagents on the ROTEM
®
system, it has to be
controlled optically if the liquid was actually aspirated while pipetting the
liquids. A typical source of error is that the pipette tip has not been
immersed into the liquid.
Like any other in vitro diagnostic system, the ROTEM
®
system requires
quality control by performing tests with standardised quality control (QC)
materials. The ROTEM
®
standardised system controls ROTROL N and
ROTROL P are based upon human plasma and will show reaction
curves at two different levels.

15 Guide ROTEM
®
Analysis - 09-2016
Performing a ROTEM
®
delta test
1. Properly attach
pin
2. Insert cup and bring
to position using the
MC rod
3. Select test, enter/
scan patient data
4. Pipetting steps are
displayed on the
screen
5. Pipetting and mixing
of reagents and blood
sample
6. Insert cup holder
in measurement
position
7. On screen display
of TEMograms and
numeric parameters
8. Discard used cup
and pin

Guide ROTEM
®
Analysis - 09-2016 16
Interpretation of ROTEM
®
thromboelastometry analysis:
examples performed on ROTEM
®
delta with liquid reagents
On each of the next pages, three typical combinations of ROTEM
®
tests
are shown. The figures are displayed exactly as on the screen of the
ROTEM
®
system. With each measurement you see the respective test
name above the parameters. In each case, the figures represent 1-4
measurements of one sample. The measurements are not commented
on the right hand page. This shall give the reader the opportunity to
reflect the interpretation and therapy on his own.
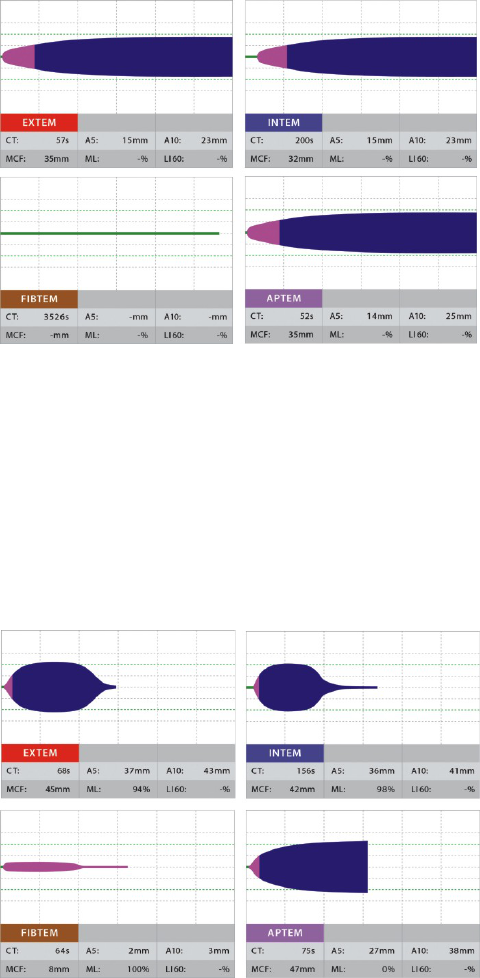
Sample 1:
normal coagulation in the ROTEM
®
. EXTEM and INTEM show a normal
coagulation activation (CT normal), normal clot formation (CFT and MCF
normal) as well as a stable clot (no lysis of the clot in EXTEM, INTEM or
FIBTEM). The FIBTEM shows a normal fibrin clot.
Should the patient bleed clinically, the following causes have to be
considered: surgical cause of bleeding, Warfarin
®
therapy (low
sensitivity of EXTEM), Aspirin
®
, clopidogrel, von Willebrand syndrome
(for these drugs respectively pathologies ROTEM
®
delta and ROTEM
®
sigma tests show low sensitivity) as well as errors (e.g. sample mix-up).
17 Guide ROTEM
®
Analysis - 09-2016
Sample 2:
strongly prolonged clot formation time (CFT), strongly reduced clot
firmness (MCF) in EXTEM and INTEM show a strongly reduced
haemostatic capacity. The zero line in FIBTEM (no clotting) shows a
strongly reduced fibrinogen level and/or a disturbed fibrin
polymerisation. The first line treatment would be a highly dosed
administration of fibrinogen concentrate (2-6 g) or cryoprecipitate or a
larger amount of FFP (5-15 units). In cases of massive bleeding it would
be considered to concomitantly transfuse platelets.
Sample 3:
fibrinolysis (lysis of the clot in EXTEM, INTEM and FIBTEM) with an at
the same time borderline acceptability of MCF (MCF = 47 mm in
APTEM). Good fibrin clot in FIBTEM. Therapy would be an
antifibrinolytic drug. In cases of persisting bleeding, administration of
platelets would be suggested (for correction of the clot formation).

Guide ROTEM
®
Analysis - 09-2016 18
Sample 4:
borderline acceptability of clot firmness in INTEM and EXTEM. No
evidence of a hyperfibrinolysis. Normal fibrin clot in FIBTEM.
Comparable results are sometimes found with or without clinical
bleeding. First line therapy for improvement of clot formation would be
the administration of platelets. In any case, the patient has typically only
a poor haemostatic reserve at further haemodilution. Depending on the
situation (further surgical blood loss expected or not), a correction of
coagulation can be considered also without the occurrence of acute
bleeding.
Sample 5:
just abnormal / still normal clot formation in EXTEM and INTEM
(depending on the investigated reference population). The relatively high
clot firmness in FIBTEM (MCF = 37 mm) can lead to a normal whole
blood coagulation, also when thrombocytopenia is present. Therefore a
blood count should be determined in this situation (in order to assess
platelet count directly), and the coagulation in course of further
haemodilution should be controlled. Patients with high fibrinogen levels
usually tolerate a thrombocytopenia better than patients with normal or
reduced fibrinogen levels. Nevertheless, it is reasonable to keep an eye
on the blood count in these situations.

19 Guide ROTEM
®
Analysis - 09-2016
Sample 6:
combined haemostasis disorder. We see a hyperfibrinolysis (lysis of the
clot in EXTEM and INTEM), a prolonged CT in INTEM (heparin effect),
a strongly reduced clot firmness in APTEM (indicates a disturbance of
clot formation exceeding fibrinolysis) as well as a zero line (no clotting)
in FIBTEM (reduced fibrinogen and / or polymerisation disorder). This
result is not compatible with clinically normal haemostasis and requires
a rapid combined treatment: an antifibrinolytic drug for the treatment of
the hyperfibrinolysis, fibrinogen or FFP (large doses) for improvement of
the clot formation. In cases of such an insufficient clot formation, a
simultaneous platelet administration is also recommended (it would
however also be possible to give fibrinogen or FFP first and then check
the clot formation).

Guide ROTEM
®
Analysis - 09-2016 20
Sample 7:
detection of heparin (strongly prolonged CT in INTEM), corrected in
HEPTEM. In this situation one can wait (short half-life of heparin) or
neutralize the heparin using protamin (during acute bleeding). As seen
in HEPTEM, the clot firmness is reduced but still within an acceptable
range. Therefore one would usually neutralise the heparin first and see
if bleeding stops. If bleeding continues, administration of FFP, fibrinogen
or platelets might be necessary.
Sample 8:
erroneous measurement. This error can occur if the pin was not attached
completely onto the axis or the cup was not inserted sufficiently into the
cup holder. Cup and pin will be in contact with each other when the cup
holder is put in place. An error message appears, the measurement
should be stopped and started again.

21 Guide ROTEM
®
Analysis - 09-2016
Sample 9:
erroneous measurement. After MCF is reached, there is a further
increase of the clot firmness after some time. An error message, which
is caused by a drying of the sample, appears. A falsely-high MCF is
detected. In this case it should be checked whether the cup holder is
dirty at its upper surface and the corresponding area on the lower side
of the instrument should be cleaned. For this, a moist cloth should be
used and no sprays should be applied on the instrument as this could
lead to damage of the ball bearing. Should there be no contamination,
the cup holder or its plastic clip might be damaged and need
replacement.
Note: test results should represent only one aspect of any
therapeutic decision. Always the situation (bleeding yes - no), the
plausibility of the findings, patient history, comorbidities as well as
the expected (surgical) course of the case has to be taken into
account. Further tests may be performed if required (PT,
antithrombin, d-dimer, platelet function tests, blood count).

Guide ROTEM
®
Analysis - 09-2016 22
Clinical cases (studied with the ROTEM
®
liquid reagents):
On the following pages, clinical cases with the corresponding ROTEM
®
analyses are shown.
Case 1:
On the right page, 3 coagulation conditions during a multiple trauma
treatment are shown.
The first test time point shows fibrinolysis in EXTEM and INTEM. In
APTEM, no lysis appears due to addition of aprotinin in the reagent.
In APTEM, we see an abnormal but still acceptable clot firmness.
The therapeutic consequence was the administration of an
antifibrinolytic drug.

23 Guide ROTEM
®
Analysis - 09-2016
The second test time point shows the therapeutic success of the
antifibrinolytic drug administration (no lysis detected any more).
Nevertheless we see a strongly reduced clot firmness (MCF) as well as
a strongly prolonged clot formation time (CFT), which was the indication
for platelet and FFP administration.
The third test time point represents a normal, whole blood coagulation
towards the end of the surgery.
Guide ROTEM
®
Analysis - 09-2016 24
Case 2:
The second case example shows the therapy control with ROTEM
®
in a
situation in which initially therapy was guided on the basis of the routine
laboratory.
We thank Dr. Georg Pfanner, consultant anaesthetist at the Department
of Anaesthesia and Critical Care of the Academic Teaching Hospital
Feldkirch, Austria ([email protected]), for recording and providing
us with this case.
The situation: a patient with a multiple trauma is admitted to the hospital.
The patient has been already notably diluted (4 l of infusions).
The initial laboratory findings show a prolonged PT (factor deficiency), a
low fibrinogen, low antithrombin and a platelet count of 101.000/l.
On the basis of these results, fibrinogen, PCC and antithrombin were
administered. Because of a strongly increased D-dimer result, the
question of an antifibrinolytic therapy aroused.
In the persistent bleeding situation, a control with ROTEM
®
is carried
out. The results show a strongly abnormal clot formation (clot firmness
reduced, clot formation time prolonged), in spite of the initial therapy.
Despite the initially acceptable platelet count, there is no sufficient whole
blood coagulation.
After therapy with fibrinogen, platelets and PCC, clinically a clear
improvement of the clinical haemostasis was found together with a
normalised whole blood coagulation in ROTEM
®
.

25 Guide ROTEM
®
Analysis - 09-2016
Initial situation:
Polytrauma => GCS 3, suspected thorax-trauma, pelvic fracture
Severe bleeding from nose, mouth, multiple wounds in the neck
Infusion therapy: HES 1000 ml, cristalloid 3500 ml
Laboratory results in hospital (65 min. after arrival):
PT 40%, aPTT 55.8s, fibrinogen 0.87 g/l, AT 49%, d-dimer 39.7,
thrombocytes 101.000/l
Assessment: reduced fibrinogen level, factor levels low, antithrombin
lowered (similar to PT), platelet count still sufficient. Fibrinolysis
suspected (very high d-dimer).
Initial therapy: 3 g fibrinogen (Haemocomplettan®), 4000 units PPSB,
3000 units antithrombin
Guide ROTEM
®
Analysis - 09-2016 26
Differential diagnosis and therapy: thromboelastometry
algorithms
On the right hand page, the differential diagnostic and therapeutic
algorithm used in the Clinic Cologne-Merheim is shown (Reference:
Vorweg M, Hartmann B, Knuttgen D, Jahn MC, Doehn M. Management
of fulminant fibrinolysis during abdominal aortic surgery. J Cardiothorac
Vasc Anesth. 2001 Dec;15(6):764-7).
This algorithm shows how coagulation activation, clot formation and
fibrinolysis are assessed starting from EXTEM and INTEM as screening
tests. If no coagulopathy is found, other reasons for the bleeding are
evaluated: a surgical bleeding or coagulopathy which is not detected by
ROTEM
®
analysis (Aspirin
®
, von Willebrand factor, warfarin?).
The combination of EXTEM and APTEM allows for a rapid detection of
a fulminant fibrinolysis.
The cause for a reduced clot firmness can be differentiated by
performing a FIBTEM test.
With the HEPTEM test, a prolonged clotting time in INTEM can be
differentiated.
Thus, many causes of acute haemostasis disorders can be recognised
rapidly and in consequence be treated appropriately.
Acknowledgement
The authors, Dr. Calatzis, Dr. Spannagl and Dr. Vorweg, would like to
thank the numerous ROTEM
®
users for their valuable discussions
during the last years, which contributed to this compendium.
We especially would like to thank and mention Prof. Dr. Wolfgang
Schobersberger, Prof. Dr. Petra Innerhofer and Dr. Dietmar Fries (A-
Innsbruck), Dr. Herbert Schöchl (A-Salzburg), Dr. Thomas Lang (D-
Hannover), Dr. Manfred Gütl (A-Graz), Prof. Dr. Sibylle Kozek-
Langenecker (A-Vienna) and Dr. Klaus Görlinger (D-Essen).

27 Guide ROTEM
®
Analysis - 09-2016
Guide ROTEM
®
Analysis - 09-2016 28
Aggregometry
Dr. Klaus Görlinger, An Ruland
Platelets are a key blood component in haemostasis. In response to a
vascular injury, they are able to adhere to the damaged vessel wall and
trigger an event that leads among others to aggregation of additional
platelets and therefore, together with other blood components, to the
formation of a stable clot.
The ROTEM
®
platelet module measures platelet aggregation
respectively via electrical impedance based on impedance
aggregrometry by Cardinal and Flower (1980).
The ROTEM
®
platelet module is an impedance aggregometer intended
for the assessment of platelet function in anticoagulated whole blood
samples. It provides quantitative and qualitative information about the
platelet aggregation by assessing the electrical impedance changes
after platelet activation with different reagents.
The ROTEM
®
platelet module is intended to be used in patients treated
with antiplatelet drugs or other drugs which may have an impact on
platelet function, as well as in patients with a suspected platelet
dysfunction due to extracorporeal circulation, trauma, sepsis or other
reasons. It is for use in clinical laboratories, hospitals or other clinical
care sites by health care professionals. The ROTEM
®
platelet module is
used in conjunction with the ROTEM
®
delta system, but does not
necessarily have to be complemented by viscoelastic testing.
Acute bleeding during or after surgery requires rapid differentiation
between surgical induced bleeding and haemostasis disorders. The
combination of the ROTEM
®
delta with the ROTEM
®
platelet and
additional diagnostic methods, considering the given limitations,
facilitates further differential treatment strategies.

29 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
platelet system
• 2 channels for simultaneous assays
• Can be used while thromboelastometry measurements are running
• Used in combination with ROTEM
®
delta
• Electronic pipetting facilitates the use outside of established
laboratories
• Ready to use single use reagents
Guide ROTEM
®
Analysis - 09-2016 30
Detection method: impedance aggregometry with ROTEM
®
platelet
Whole blood is pipetted into a cuvette containing a stirring bar and
special electrodes, energized with a certain voltage. Before inducing
platelet aggregation, an impedance baseline is determined. After adding
aggregating agents, the platelets are activated and start to aggregate.
The increase of the electrical impedance is measured over
the time of aggregation. It is directly proportional to the extent of platelets
involved in coating the electrodes by aggregation. The results of the
measurement are processed with a specific software.
The parameters of ROTEM
®
platelet analysis
AUC (Area under the curve):
The AUC represents the area under the aggregation curve from
the start of the measurement until 6 minutes of runtime.
AUC reflects the overall platelet aggregation.
MS (Maximum Slope):
The MS is the maximum slope to the aggregation graph.
MS is a measure for the rate of aggregation.
A6 (Amplitude at 6 minutes):
The A6 reflects the measured impedance, 6 minutes after
starting the test.
A6 is a measure for the extent of platelet aggregation.

31 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
platelet detection method
ROTEM
®
platelet parameters and scaling

Guide ROTEM
®
Analysis - 09-2016 32
ROTEM
®
platelet tests
The ROTEM
®
platelet analysis extends the resulting diagnostic power
by a number of additional tests and parameters, which
• allow differentiation between different platelet drug effects on platelet
aggregation.
• allow to detect platelet dysfunction due to e.g. extracorporeal assist
devices, surgery...
1
In ARATEM, the platelets are activated with arachidonic acid. Platelet
function is assessed for example in patients treated with
cyclooxygenase inhibitors (e.g. acetylsalicylic acid).
In ADPTEM, the platelets are activated with adenosine diphosphate.
Platelet function is assessed for example in patients treated with ADP
receptor antagonists (e.g. clopidogrel).
In TRAPTEM, the platelets are activated with thrombin receptor
activating peptide. Platelet function is assessed for example in patients
treated with PAR-1 receptor antagonists (e.g. vorapaxar) or GP IIb/IIIa
receptor antagonists (e.g. abciximab).
1.Petricevic M.et al.; Bleeding risk assessment in patients undergoing elective
cardiac surgery using ROTEM
®
platelet and Multiplate
®
impedance
aggregometry. Anaesthesia 2016 Jun; 71(6):636-47

33 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
platelet tests
ARATEM:
platelet activation
with arachidonic acid
ADPTEM:
platelet activation
with ADP
TRAPTEM:
platelet activation
with TRAP

Guide ROTEM
®
Analysis - 09-2016 34
ROTEM
®
platelet expected values
On the opposite page, typical ROTEM
®
aggregometry values are
shown, which are found when healthy patients without aggregation
disorders who are not taking antiplatelet drugs are analysed. Depending
on the examined population, these values can vary. It is therefore
recommended, at implementation of ROTEM
®
platelet, to analyse some
patients without pathological findings in order to establish respective
'local' reference ranges.
The reference values for the different tests depend on the sample type
used (citrate, heparin or hirudin sample tubes).
Interpretation of ARATEM, ADPTEM, TRAPTEM
In case of impaired platelet function, several parameters (AUC, A6 and
MS) are reduced. The cause of platelet impairment may be anti-platelet
or other influencing drug intake or non-drug induced (e.g. due to
cardiopulmonary bypass, extracorporeal assist devises, trauma,
surgery, infection or sepsis or in cases of thrombocytopenia).
Non-drug induced platelet dysfunction may be detected on all tests
(ARATEM, ADPTEM and TRAPTEM). However, in sepsis
1
for example,
the effect may be more pronounced on the ADPTEM test and in trauma
2
on the TRAPTEM test. Drug induced platelet dysfunction may show
different test patterns depending on the drug (see below, page 37).
1.Adamzik M. et al.; Whole blood impedance aggregometry as a biomarker for the
diagnosis and prognosis of severe sepsis. Crit. Care. 2012 Oct 22; 16(5):R204
2.Chapman MP et al.; Early TRAP pathway platelet inhibition predicts
coagulopathic hemorrhage in trauma. Shock.2015; 43 (6 Suppl1):33

35 Guide ROTEM
®
Analysis - 09-2016
ROTEM
®
platelet preliminary reference ranges
For citrated samples
For heparinized samples
For hirudinized samples
Test
/ Parameter
AUC
(Ohm*min)
A6
(Ohm)
MS
(Ohm/min)
ADPTEM (n=20) 56-139 16-38 4-11
TRAPTEM (n=175) 61-156 15-36 5-14
ARATEM (n=20) 70-153 19-41 6-13
Test
/ Parameter
AUC
(Ohm*min)
A6
(Ohm)
MS
(Ohm/min)
ADPTEM (n=20) 57-133 16-34 4-12
TRAPTEM (n=60) 66-169 15-38 6-18
ARATEM (n=20) 69-144 17-37 6-14
Test
/ Parameter
AUC
(Ohm*min)
A6
(Ohm)
MS
(Ohm/min)
ADPTEM (n=20) 86-201 23-52 7-17
TRAPTEM (n=60) 67-155 17-37 6-15
ARATEM (n=20) 84-193 21-52 7-16
Guide ROTEM
®
Analysis - 09-2016 36
Assessment of the ROTEM
®
platelet analysis
Drug induced or non-drug induced platelet dysfunction is detected by
decreased AUC, A6 and MS test results.
A. In patients treated with cyclooxygenase inhibitors (e.g. acetylsalicylic
acid) platelet aggregation may be impaired. This will be detected in the
ARATEM test.
B. In patients treated with ADP receptor antagonists (e.g. clopidogrel)
platelet aggregation may be impaired. This will be detected in the
ADPTEM test.
C. In patients treated with dual antiplatelet therapy (e.g. acetylsalicylic
acid and clopidogrel) platelet aggregation may be impaired. This will be
detected in ADPTEM and ARATEM.
D. In patients treated with PAR-1 receptor antagonists (e.g. vorapaxar)
platelet aggregation may be impaired. This will be detected in
TRAPTEM.
E. In patients treated with GP IIb/IIIa inhibitors (e.g. abciximab) platelet
aggregation may be impaired. This will be detected in all tests: ADPTEM,
TRAPTEM and ARATEM.
Non-drug induced platelet dysfunction may also show impaired platelet
aggregation (e.g. due to cardiopulmonary bypass, extracorporeal assist
devises, trauma, surgery, infection or sepsis or in cases of
thrombocytopenia). This may be detected in all tests: ADPTEM,
TRAPTEM and ARATEM. However, in sepsis for example, the effect
may be more pronounced on the ADPTEM test and in trauma on the
TRAPTEM test.
Limitations
Low platelet count may show abnormal aggregation.

37 Guide ROTEM
®
Analysis - 09-2016
Assessment of the ROTEM
®
platelet analysis
Examples of impaired platelet aggregation after drug intake.
A. Patient treated with acetylsalicylic acid:
B. Patient treated with clopidogrel:
C. Patient treated with clopidogrel and acetylsalicylic acid:
D. Patient treated with vorapaxar:
E. Patient treated with abciximab:
Guide ROTEM
®
Analysis - 09-2016 38
Assessment of the ROTEM
®
platelet analysis
As in all diagnostic tests, correct pre-analytics and correct performance
of the assay are essential for meaningful results.
“Correct sampling“ means: Complete filling of the sampling tube (in order
to ensure the correct anticoagulant-blood ratio); avoidance of
haemolysis during sampling (prevent excessive stasis, use of a needle
with sufficiently wide diameter).
Three sample tube types may be used: heparin, citrate and hirudin.
Normal values have been established for each sample type in
combination with all available tests. Citrated samples are not
recommended in situations where the patient‘s blood may contain
heparin. According to the chosen anticoagulant in the sample tube, the
corresponding sample resting time (between 2-30 min.) needs to be
observed (refer to the reagents‘ instructions of use).
A pneumatic tube transportation system may influence the sample‘s
platelets. As a precaution, this should be verified (split the sample and
analyse with/without transport by the tube system).
The samples should be stored at room temperature until analysis.
ROTEM
®
platelet analysis should be performed within two hours after
blood sampling.
The steps to be performed for ROTEM
®
platelet analysis are shown on
the right. The test operation is generally simple - also for staff without any
laboratory experience. Nevertheless, a certain familiarisation period and
motivation are necessary.
Apart from the correct performance of the analysis – as in every
laboratory test – the plausibility control of the analysis is important.
Measurements with irregular shapes (e.g. noised curves), generally
accompanied by error messages, should be repeated.
In case of unexpected results, a normal donor sample with known
aggregation level should be tested to verify system integrity. The normal
donor should not have ingested acetylsalicylic acid or clopidogrel or
acetylsalicylic acid- or clopidogrel-containing compounds in the
preceding 10 days.

39 Guide ROTEM
®
Analysis - 09-2016
Performing a ROTEM
®
platelet test
1. Insert the cuvette 2. Select test, enter/
scan patient data
3. Pipetting steps
are displayed on the
screen
4. Pipetting of
diluent, blood and
reagent
5. On screen display of
graphs and numeric
parameters
6. Discard used
cuvette

Guide ROTEM
®
Analysis - 09-2016 40
Differential diagnosis and therapy: algorithms
Acute bleeding during or after surgery requires rapid differentiation
between surgical induced bleeding and haemostasis disorders. The
combination of the ROTEM
®
delta with the ROTEM
®
platelet and
additional diagnostic methods, considering the given limitations,
facilitates further differential treatment strategies.
Patient drug history
ROTEM
®
delta
analysis
Bleeding patient
ROTEM
®
platelet
analysis
Abnormal
Abnormal Normal Normal
Consider
surgical bleeding
Haemostasis disorder:
Coagulopathy
(or thrombocytopenia)
Haemostasis disorder:
Platelet dysfunction
(or thrombocytopenia)

41 Guide ROTEM
®
Analysis - 09-2016
Differential diagnosis and therapy: algorithm example
Severe Bleeding Algorithm
Diffuse bleeding and
blood transfusion considered
A5
EX
< 35 mm or
CT
FIB
> 600 s or
ML
!
5% (within 60 min)
Tranexamic acid
25 mg/kg as a single
bolus
(if not already given prophylactically)
A5
EX
< 35 mm
and
A5
FIB
< 8 mm (12 mm)
Fibrinogen concentrate
or Cryoprecipitate
(dose cal.)
Target: A5
FIB
≥ 12 mm
(16 mm)
A5
EX
< 35 mm and
A5
FIB
≥ 8 mm (12 mm)
or platelet dysfunction
Platelet concentrate
1-2 pooled or
apheresis
1
CT
EX
> 80 s
PCC 15-25 IU / kg bw
or
FFP 10-15 mL / kg bw
CT
IN
> 240 s CT
HEP
> 240 s
Consider FFP
10-15 mL / kg bw
Consider Protamine
25-50 mg (2.5-5 mL)
Re-check after 10-15 min
using a new blood sample
Ongoing bleeding
YES
YES
YES
YES
YES
YES
YES
YES
NO
NO
NO
NO
NO
NO
DONE
Hemorrhagic shock or
BE < -6 mmol/L or
Hb < 10 g/dL or
ISS ! 25 or
TASH-Score ! 15
YES
CHECK
NO
1
Platelet concentrate (PC) transfusion:
Check platelet function with ROTEM® platelet (ADPTEM and TRAPTEM)
Consider tranexamic acid (25 mg/kg) and/or desmopressin (DDAVP; 0.3µg/kg)
in patients with dual antiplatelet therapy and/or ADPTEM < 30 Ω·min
Expected increase per pooled/apheresis PC per 80 kg: 8-10 mm in A5
EX
→
A5
EX
< 35 mm (or ADPTEM < 30 Ω·min): 1 pooled or apheresis PC
A5
EX
< 25 mm (or ADPTEM < 30 Ω·min and TRAPTEM < 50 Ω·min): 2 pooled or apheresis PC
A5
EX
< 15 mm: 2 platelet concentrates + fibrinogen substitution (≥ 4 g)
Dr. Andreas Calatzis, PD Dr. med. Michael Spannagl
Department for haemostasis and transfusion medicine
University Clinic, Ludwig-Maximilian University
Ziemssen Str. 1, 80336 Munich, Germany
Dr. med. Matthias Vorweg
Cologne's City Hospitals gGmbH
OP Management
Ostmerheimer Str. 200, 51109 Cologne, Germany
Dr. med. Klaus Görlinger
Medical director - TEM international GmbH
Martin-Kollar-Str. 13-15, 81829 Munich, Germany
An Ruland
Product Manager - TEM international GmbH
Martin-Kollar-Str. 13-15, 81829 Munich, Germany
The ROTEM
®
system is an improvement of thromboelastography as
described by Professor Helmut Hartert. Andreas Calatzis developed the
ROTEM
®
system in collaboration with the physicist Pablo Fritzsche.
Michael Spannagl is a consultant for internal medicine and angiology. He
has been working for many years on the diagnosis and management of
acute and chronic disturbances of the haemostatic system. Matthias
Vorweg, University Hospital Bonn, has introduced ROTEM
®
analysis in
the anaesthesiology department of the Cologne-Merheim hospital more
than 10 years ago. The Cologne-Merheim hospital was one of the first
centres to introduce the concept of the ROTEM
®
based differential
diagnosis and targeted therapy in the clinical routine. Klaus Görlinger
was the ROTEM
®
pioneer in the Department of Anaesthesiology of the
University Hospital Essen. One of his major fields of expertise is the
development and implementation of Point of Care (POC) guided
algorithms for goal directed coagulopathy management. Dr. Görlinger is
now the medical director of TEM international in Munich, Germany.
ROTEM
®
is a registered trademark of Tem Innovations GmbH, Munich, Germany.
Manufacturer:
Tem Innovations GmbH
Martin-Kollar-Str. 13-15
D-81829 Munich
Phone +49 (89) 45 42 95-0
Fax +49 (89) 45 42 95 22
